Applications
Spheroid and Organoid Sorting & Isolation
Unlocking the Power of 3D Cellular Models in Drug Discovery
Spheroids and organoids, collectively referred to as 3D cellular models, are rapidly transforming the landscape of preclinical drug development. Unlike traditional 2D cell cultures, these 3D systems more accurately mimic the structural complexity, microenvironment, and functional responses of human and animal tissues. This makes them far more predictive of clinical outcomes, and increasingly attractive for pharmaceutical research. Regulatory agencies such as the FDA and NIH are actively encouraging the adoption of these models, acknowledging the poor predictability of animal testing in assessing drug efficacy and toxicity.
However, the promise of spheroids and organoids comes with challenges. A major obstacle is their heterogeneity: populations of 3D models often vary widely in size, morphology, and functionality. This variability introduces noise into assays, reduces reproducibility, and can lead to misleading results. For drug developers, the consequence is significant, false negatives or positives that inflate costs and increase the risk of failed clinical trials.
By enabling standardization, phenotypic analysis, and single model -omics, spheroid and organoid sorting and isolation addresses these challenges head-on, improving reproducibility, reducing risk, and paving the way for more predictive and cost-effective drug discovery.
You're in good company
These leading institutions use our single cell and 3D cell model technologies:









Spheroid applications
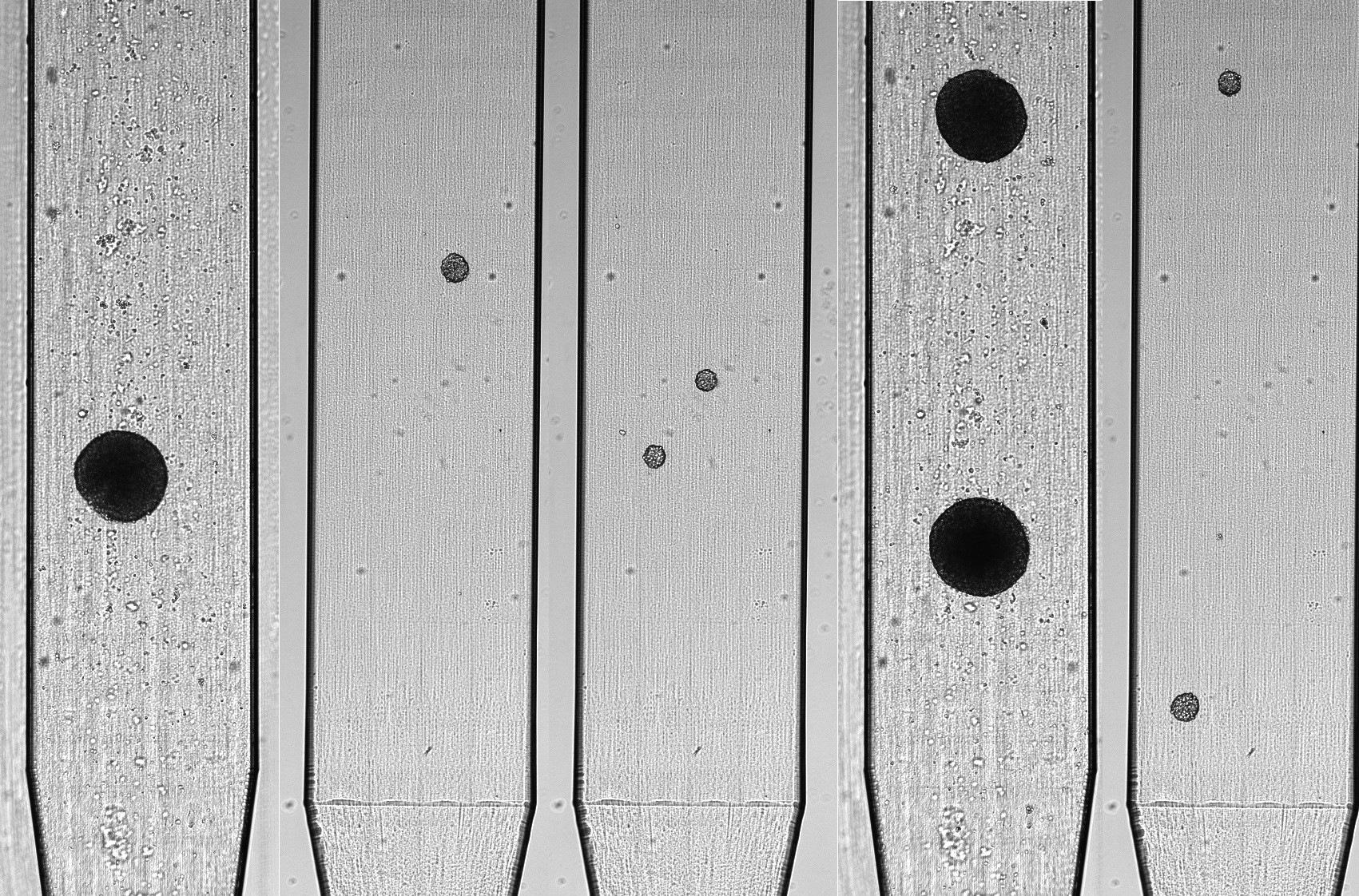
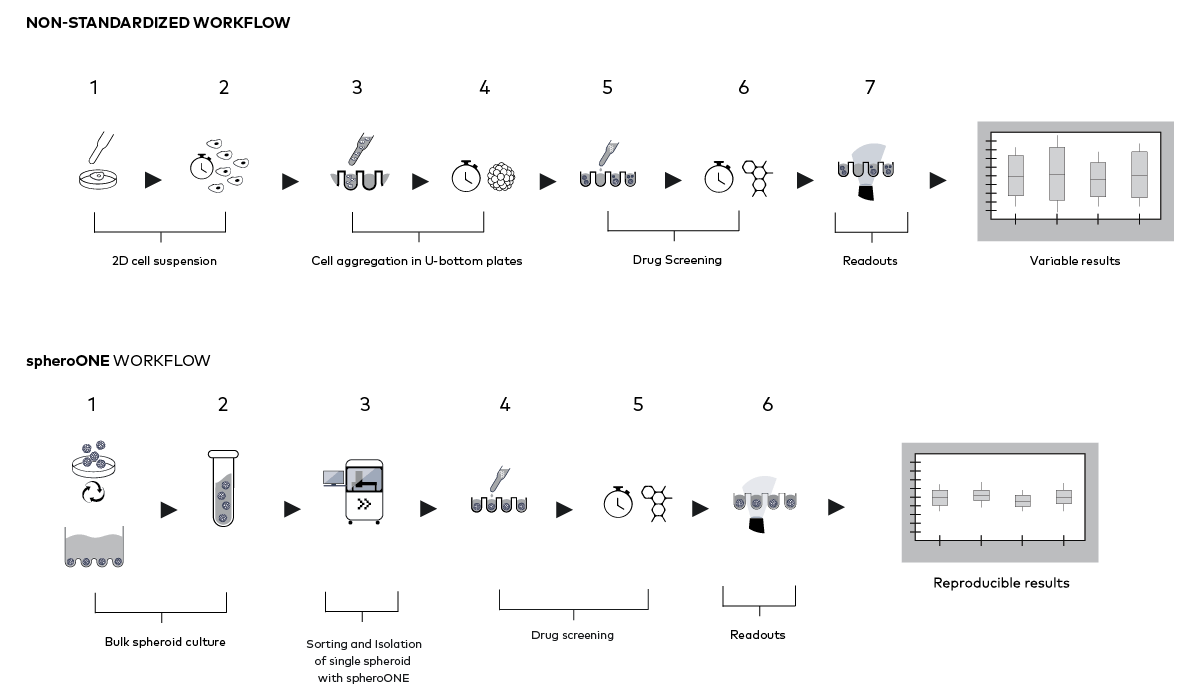
STANDARDIZATION OF 3D MODELS FOR DRUG TESTING
Standardizing of 3D cellular models before drug testing reduces variability and improves assay reproducibility.
LINKING PHENOTYPE TO MECHANISM IN DRUG RESPONSE
Sorting spheroids and organoids after drug treatment enables phenotypic analysis, distinguishing resistant from sensitive populations for deeper biological insights.

SINGLE -OID OMICS FOR DEEPER BIOLOGICAL INSIGHTS
Isolating individual spheroids or organoids allows single 3D model genomics, transcriptomics, proteomics, or metabolomics.

SINGLE -OID OMICS FOR DEEPER BIOLOGICAL INSIGHTS
Precision positioning of 3D cellular models enables reproducible high-content imaging, accurate co-culture setups, and standardized readouts for drug discovery.
Standardized 3D cell-model drug discovery workflow
Featured resources
POSTER
Drug response profiling of individual primary colorectal cancer tumoroids using a novel automation workflow and Al-assisted image analysis
Here we present a workflow combining automated sorting and isolation of tumoroids with spheroONE and integrated systems for fluid handling, staining, imaging, and AI-based analysis. This approach enables reliable viability and proliferation assays on both established cell line–derived spheroids (HTC116) and patient-derived CRC tumoroids after drug treatment.
By uniting automation, precise reagent handling, and high-content imaging, the workflow improves standardization of 3D model assays, supports flexible drug testing readouts, and advances applications in disease modelling, personalized medicine, and translational drug discovery.
Application Note
Preparation of HepaRGTM spheroid assay-ready plates using spheroONE
HepaRG™ cells are unique bipotent human cells that can differentiate into both hepatocyte-like and biliary-like cells. They express key detoxifying enzymes and are widely used in liver toxicity assays during early drug discovery.
In this study, we demonstrate efficient formation of functional HepaRG™ spheroids and their precise sorting and isolation with spheroONE. The workflow enables assay-ready plates with homogeneous, single spheroids per well, improving reproducibility and reliability in preclinical liver toxicity testing.
VIDEO
Growth dynamics of spheroONE-isolated HEK-GFP spheroids during camptothecin treatment.
Camptothecin, used as a chemotherapeutic agent, blocks DNA replication and induces apoptosis in dividing cells. In this assay, only spheroids exposed to the lowest camptothecin concentrations continue to grow, while higher doses suppress proliferation. Because spheroids were isolated as single, uniformly positioned with spheroONE, growth dynamics could be easily monitored by live imaging over time.

